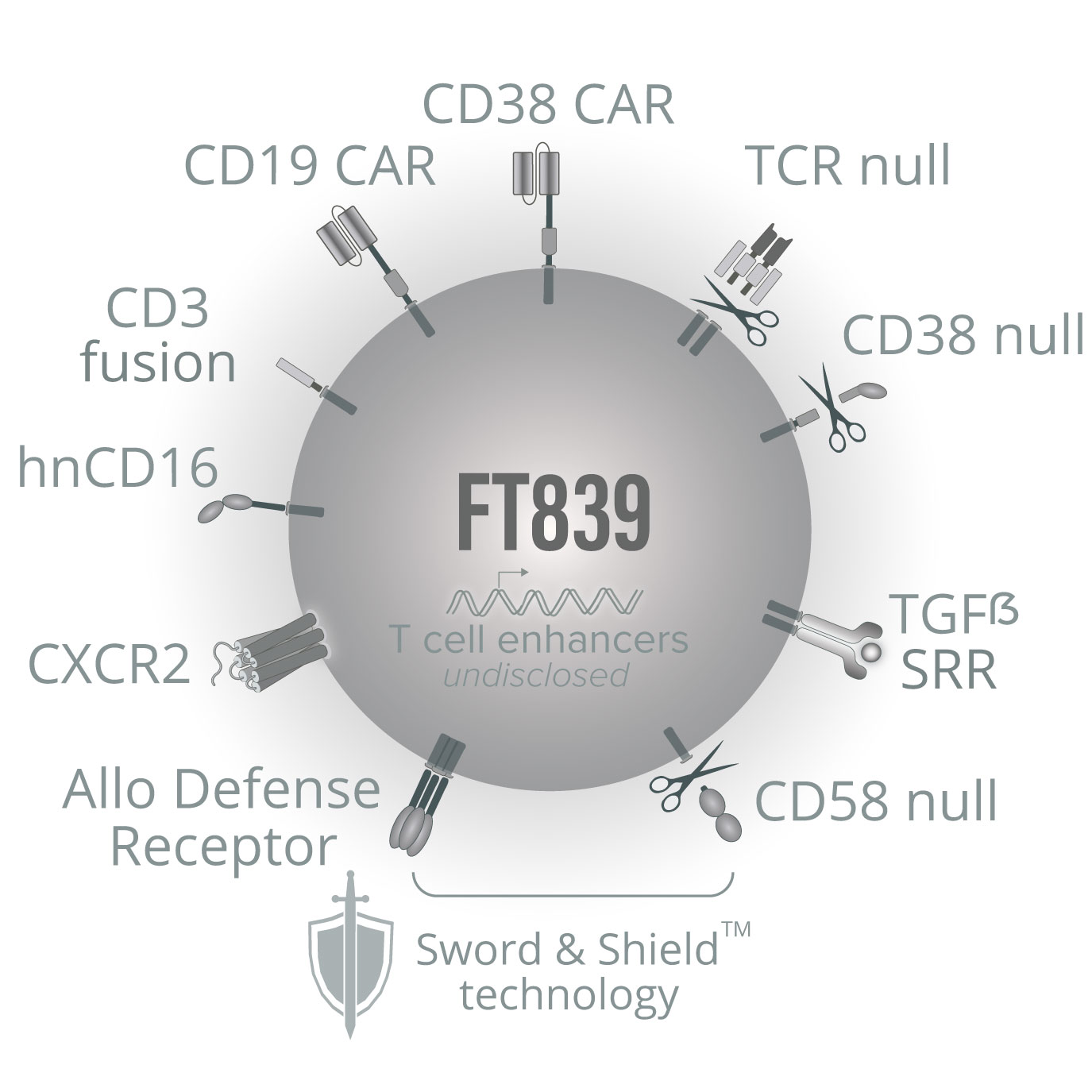
FT839
We are applying our iPSC product platform to preclinically develop next-generation CAR T-cell therapies with the potential to target multiple aberrant cell types while evading recognition by alloreactive immune cells. FT839 is our first multi-antigen CAR T-cell product candidate that is designed to express two unique CARs: a first CAR targeting CD19+ B cells, and a second CAR targeting additional disease-causing cells. At the 2024 American Society of Hematology (ASH) Annual Meeting, we demonstrated that iPSC-derived CAR T cells targeting CD19 and the cell surface glycoprotein CD38 specifically eliminated a variety of malignant cell types, including CD19+ lymphoma and CD38+ multiple myeloma cell lines, in several in vitro cytotoxicity assays and in vivo models. In addition, dual CAR T cells showed enhanced elimination of CD19+CD38+ cells in vivo compared to single CAR controls. In a preclinical proof-of-concept study in autoimmunity, using unmatched PBMCs sourced from a patient with SLE, dual CAR T cells showed robust eradication in vitro of aberrant CD19+ B cells, CD38+ plasma cells, and CD38+ activated T cells.