FT825
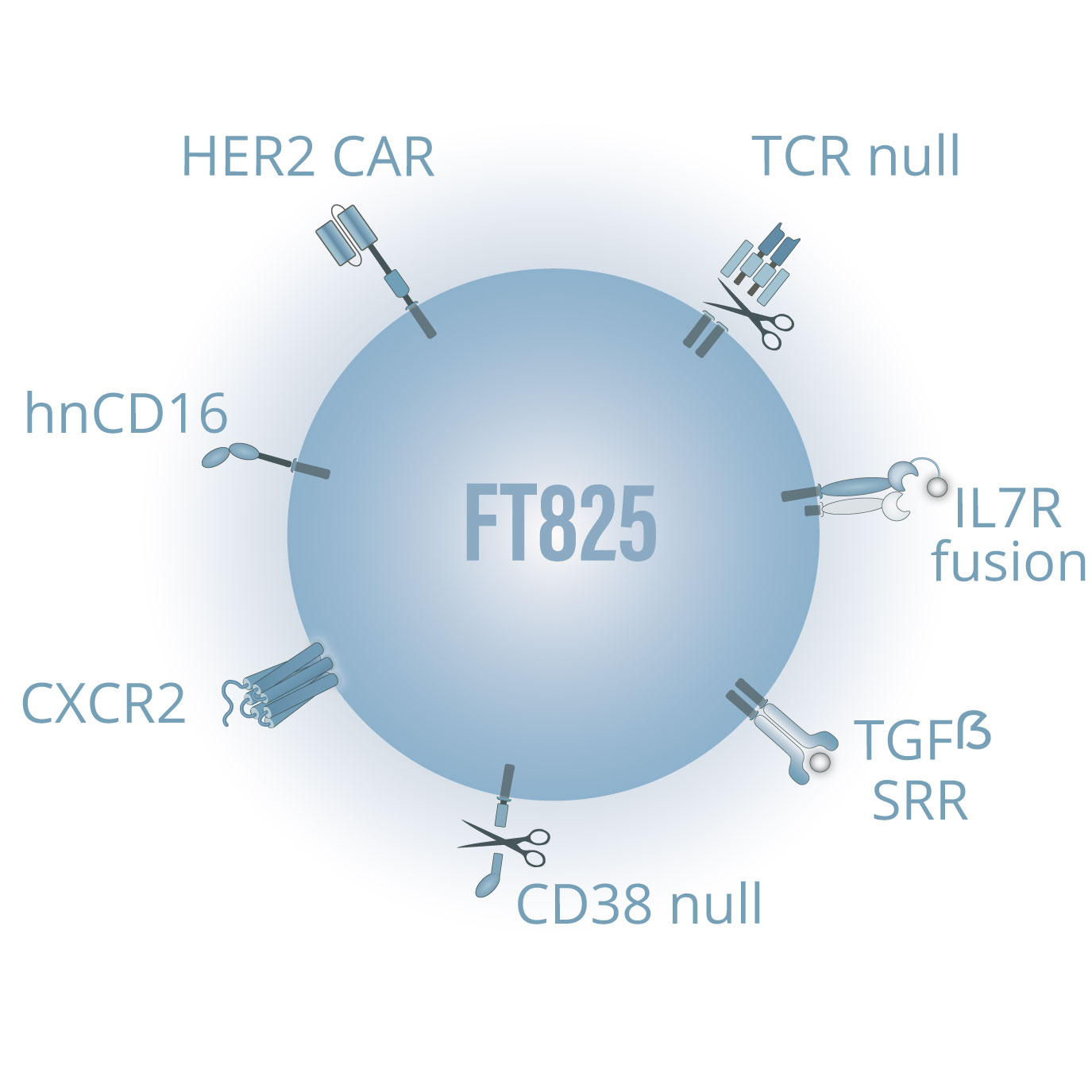
FT825 is our most advanced CAR T-cell product candidate for the treatment of solid tumors, which is currently undergoing Phase 1 clinical investigation in collaboration with Ono Pharmaceutical. FT825 is an iPSC-derived, CAR T-cell product candidate targeting human epidermal growth factor receptor 2 (HER2)-expressing solid tumors, and is specifically designed to overcome challenges in treating solid tumors, such as breast, gastric, bladder, and lung cancers.
To find out more about our clinical trials click here.