FT836
FT836 is our a multiplexed-engineered CAR T-cell product candidate targeting major histocompatibility complex (MHC) proteins A (MICA) and B (MICB). The expression of MICA/B cell-surface proteins is induced by cellular stress or malignant transformation, and is detectable across many types of cancer cells with limited expression on healthy tissue. MICA/B targeting is emerging as a novel cancer-specific strategy to attack a wide range of solid tumors, however, proteolytic cleavage and shedding of MICA/B at the membrane-proximal α3 domain is a common mechanism of tumor escape. FT836 is designed to uniquely target and bind the α3 domain, which has been shown to stabilize MICA/B expression and induce robust cytolytic killing of tumor cells.
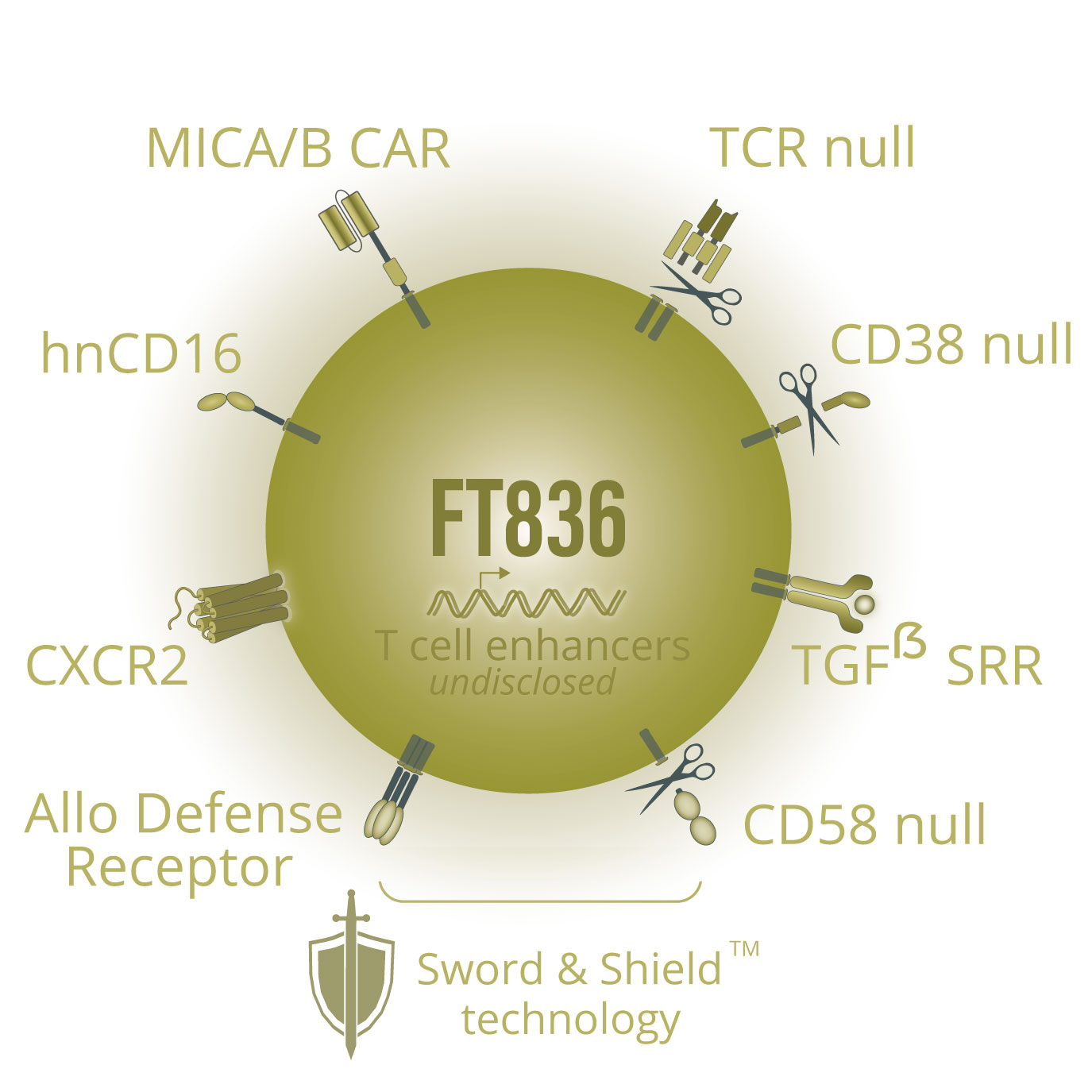
At the 2024 Society of Immunotherapy of Cancer (SITC) 39th Annual Meeting, we presented preclinical data showing that FT836 exerted potent and durable anti-tumor activity in vivoacross a broad array of solid tumors (see figure below). In addition, treatment of tumor cells with chemotherapy or radiation therapy in vitro elicited an increase in MICA/B expression and further enhanced the cytolytic activity of FT836, indicating the potential for combination with standard-of-care regimens used for the treatment of solid tumors.
FT836 is also our first product candidate to incorporate our novel Sword & Shield technology, which utilizes our ADR technology alongside the complete knock-out of CD58 (CD58KO), to both target and evade host alloreactive immune cells. In preclinical studies presented at SITC, iPSC-derived Sword & Shield CAR T cells demonstrated functional persistence and durable anti-tumor activity in vivo that was uniquely maintained upon supraphysiological challenge with alloreactive T cells, indicating the potential of Sword & Shield CAR T cells to thrive without administration of conditioning chemotherapy to deplete host immune cells.